
Ultratech’s Quality Assurance teams function independently for strict monitoring & control of various activities of the plant as per the pre-defined processes and procedures. High quality performance standards are achieved through qualified and experienced technical man power, sophisticated equipments, documented procedures and an established quality assurance system.
Ultratech is committed to total quality management and ensures that ICH guidelines and cGMP standards are complied and products comply with international quality standards.
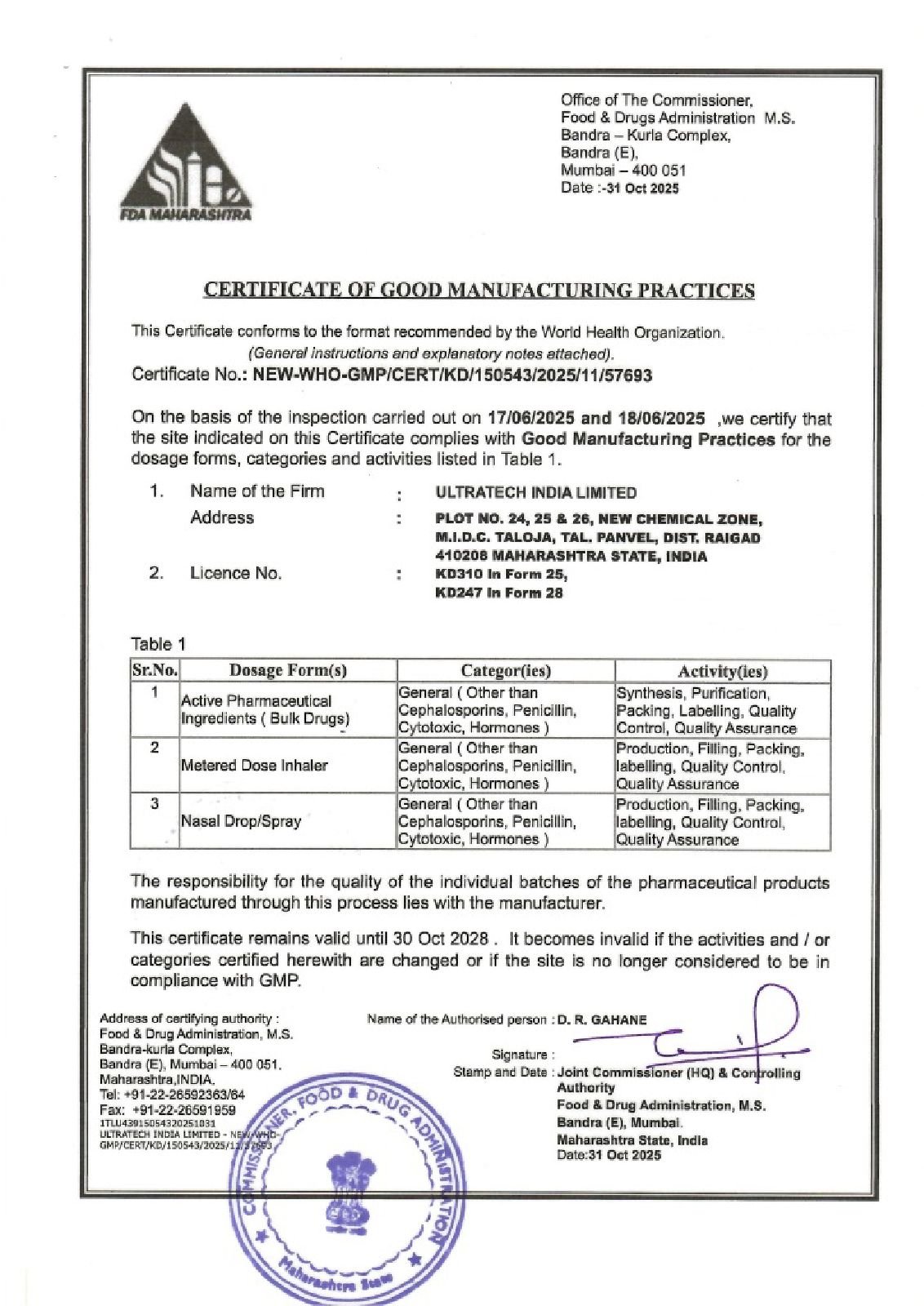
Regulatory compliance
Utratech: WHO GMP Certified. Global Quality Assured
Holding the prestigious WHO cGMP certification, we embed global best practices into our operations. The foundation of our success is a robust Quality Management System (QMS) designed for meticulous control and continuous improvement. All our products are manufactured under strict Quality standards, employing rigorous processes, advanced controls, and exhaustive testing to ensure consistency and efficacy in every batch. We are committed to delivering the highest caliber of pharmaceutical products

Our facility is HACCP Certified
Our facility is HACCP Certified, signifying a rigorous, proactive approach to product safety. This internationally recognized system ensures every stage of our production, from raw materials to final product, is meticulously analyzed for hazards and controlled at critical points. We guarantee consistent quality and reliability in every batch

Ultratech is ISO 9001-2015, ISO 14001:2015 and GMP certified
The practices at Ultratech are adherence to latest quality & safety standards. We ensure the implementation of cGMP standards, follow our in-house SOPs, and pharmacopoeial specifications. Most of our products are supported with technical packages comprising of Drug Master files, Product Dossiers, Free sale certificates, Certificate of Pharmaceutical Products, etc.
'Excellence in Quality' is the Voice of every member of Ultratech India Ltd.
ISO 9001-2015
Quality Mangement System Certificate

Environmental Compliance
The intermediates, wastes and effluents are treated effectively so as to ensure compliance with environmental norms. Our environmental policy is to produce highest quality products in harmony with the ecological system and to minimize the damage to the environment by complying with all the statutory regulations and ISO-14001 standards.
ISO 14001-2015
Environmental Management System Certificate

